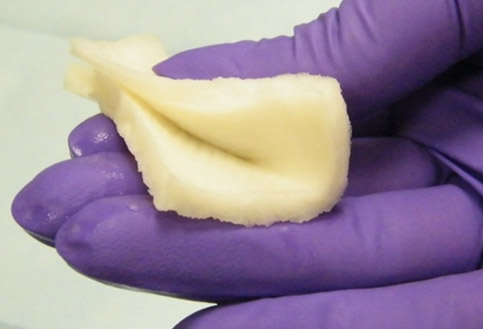
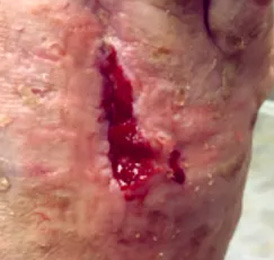
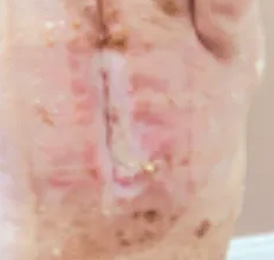
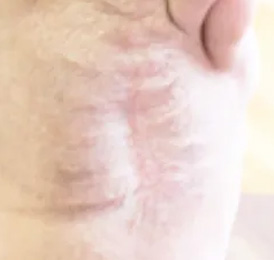
bio-ConneKt® Wound Matrix
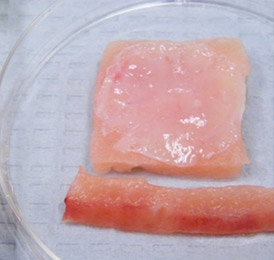
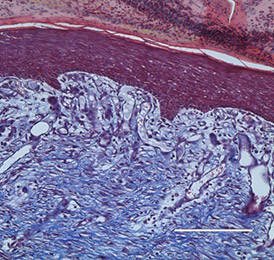
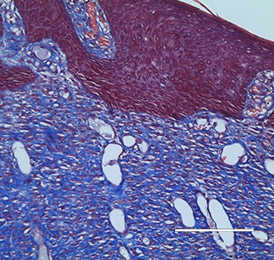
The bio-ConneKt® Wound Matrix is a collagen-based wound dressing for the local management of moderately to heavily exuding wounds. It is indicated for use wherever there is breach of the skin. Some of the common types of wounds where bio-ConneKt® Wound Matrix has been used include: full and partial thickness wounds, draining wounds, tunneling wounds, pressure sores/ulcers, venous ulcers, chronic vascular ulcers, diabetic ulcers, trauma wounds (e.g. abrasions, lacerations, partial thickness burns, skin tears), and surgical wounds (e.g. donor sites/grafts, post-laser surgery, post-Mohs’ surgery, podiatric wounds, dehisced surgical incisions).